Their decision came despite strong pressure from the Roman Catholic Church and a clear lack of enthusiasm from the right-wing coalition government of Prime Minister Silvio Berlusconi, ANSA reported.
Bishop Elio Sgreccia, vice president of the Pontifical Academy for Life, warned Thursday that any doctor or woman, or anyone involved in promoting the drug, would be subject to excommunication from the church.
"One takes a decision as if one was dealing with any anti-fever drug and not a means of suppressing a life, even if it is at its early stage," said junior interior minister Alfredo Mantovano.
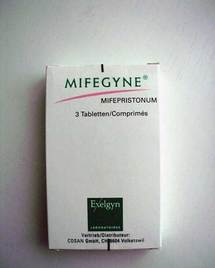
AIFA's board decided to grant the November 2007 request of the French laboratory Exelgyn to put the drug on the market. Mifegyne has been available in France since 1988.
RU486, which is not to be confused with the so-called "morning after" pill Norlevo, which has been available in Italy since 2000, allows the user to have a chemically induced abortion instead of a surgical intervention.
The pill is used to terminate an unwanted pregnancy within the first five or seven weeks, depending on the country where it is prescribed.
A 1978 Italian law allows surgical abortion only.
Italian law also allows doctors to refuse to perform abortions as conscientious objectors -- an option used by some 70 percent of Italy's gynaecologists.
----------------------------------------------------------------------------------------------------------------------------
Bishop Elio Sgreccia, vice president of the Pontifical Academy for Life, warned Thursday that any doctor or woman, or anyone involved in promoting the drug, would be subject to excommunication from the church.
"One takes a decision as if one was dealing with any anti-fever drug and not a means of suppressing a life, even if it is at its early stage," said junior interior minister Alfredo Mantovano.
AIFA's board decided to grant the November 2007 request of the French laboratory Exelgyn to put the drug on the market. Mifegyne has been available in France since 1988.
RU486, which is not to be confused with the so-called "morning after" pill Norlevo, which has been available in Italy since 2000, allows the user to have a chemically induced abortion instead of a surgical intervention.
The pill is used to terminate an unwanted pregnancy within the first five or seven weeks, depending on the country where it is prescribed.
A 1978 Italian law allows surgical abortion only.
Italian law also allows doctors to refuse to perform abortions as conscientious objectors -- an option used by some 70 percent of Italy's gynaecologists.
----------------------------------------------------------------------------------------------------------------------------









 Home
Home Politics
Politics









